What is the solution in chemistry and definition of the solution? How many types of solutions are presents etc.
SOLUTIONS
Chemical:
Any substance that is present in the lab shelf, in its original container & not involved in any reaction or process.
Reagent:
Chemical solution of a certain
concentration (%age Normal, Molar, etc.) that is present in the lab, being
used directly in the reaction or process
Solution:
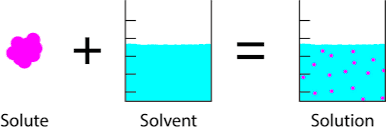
A homogeneous mixture that has the same chemical composition and physical properties everywhere is called a solution. The solution has two main parts;
Solvent:
Larger amount in solution e.g. Water in
sugar solution
Solute:
Lesser amount in solution e.g. sugar in
sugar solution
Solvation/Hydration:
Interaction
between solvent and solute in a solution called solvation. If H2O
is used as a solvent then the solvation process will be termed Hydration.
STRENGTH OF SOLUTIONS:
Parts per million (ppm):
The number of milligrams in one liter of
solution is the number of ppm solution, as 10mg in 1000ml of distilled water will
be 10ppm solution
Normality:
Gram equivalent weight of the substance in 1L of the solution is 1 Normal (1N) solution
Molarity:
1 molar weight in one-liter solution is 1 Molar (1M) solution.
Molality:
1 molar weight in one liter of solvent is 1 Molal (1m) solution.
Percent solution: the amount of solute in 100 ml solution.
Types of percent solutions
1. Volume/volume
2. Weight/volume
3. Weight/weight
VARIOUS FORMULAE USED DURING SOLUTION PREPARATION:
1. N1V1=N2V2
2. Volume = weight
/specific gravity
3. Actual volume = volume x 100 / purity